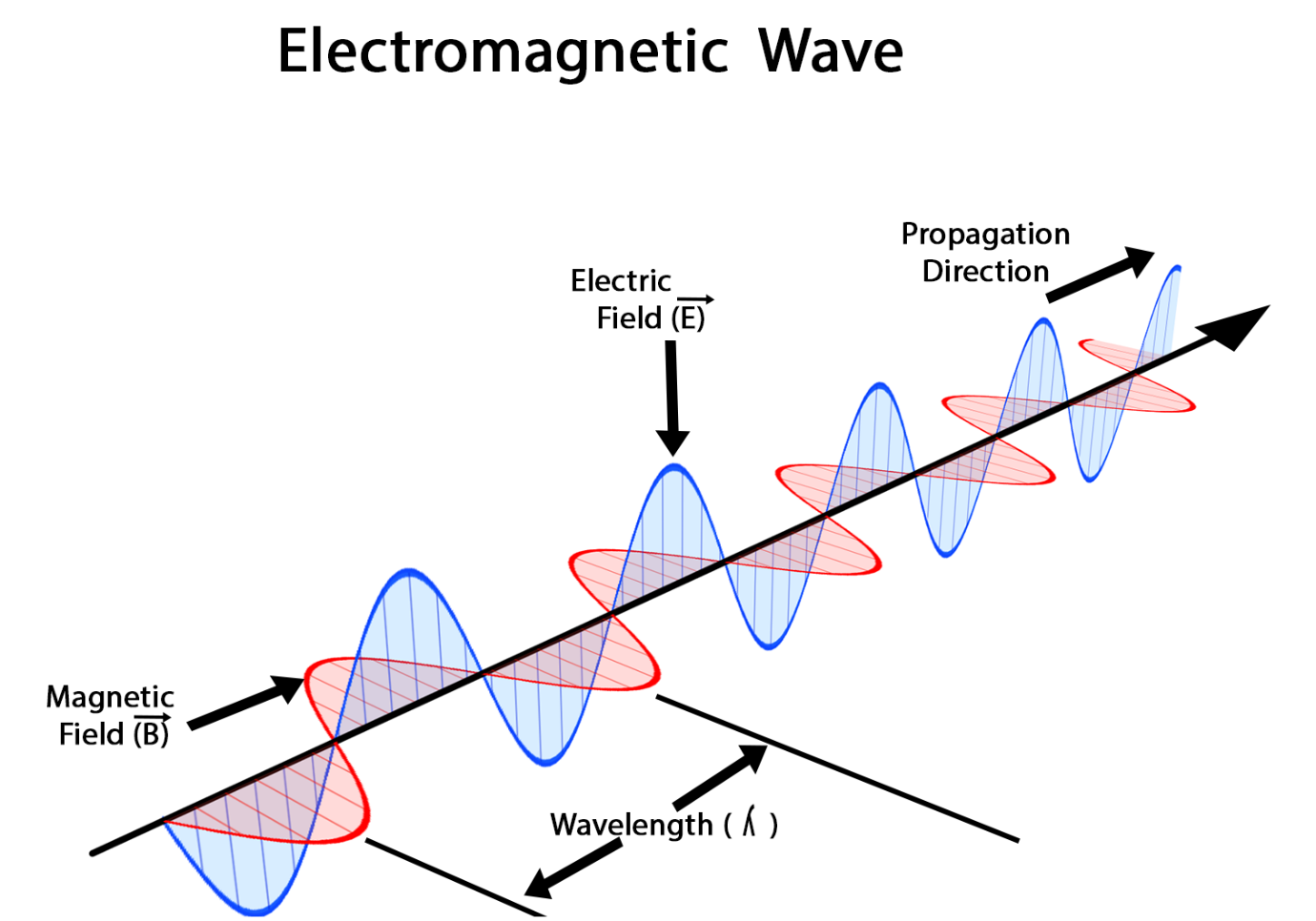
What Are The Principles Of Electromagnetic Wave Propagation In Electrical Engineering?
Properties are an integral concept of chemistry, and students often come across several important properties, including chemical, physical, and thermodynamic properties. If you're a student of class 12 studying chemistry, you must be aware of the importance of grasping these properties to excel in your exams. To ensure that you don't falter in your exams, it is crucial to understand all the properties well.
However, it's important to note that not all statements about properties are true. In fact, some statements about properties are false, and it's important to identify them to avoid any confusion while studying. In this post, we'll be discussing the false statements about properties for class 12 students.
One of the most common false statements about properties is that physical properties of matter are unchangeable and can't be modified. This is not entirely true. While some physical properties remain constant, certain processes can cause modifications in them, such as changes in temperature or pressure.
Another false statement you might come across is that chemical properties can be observed without any chemical reactions taking place. This statement is not accurate as chemical properties are only observed when a chemical reaction takes place. Chemical properties are inherent and are not visible until an appropriate reaction is initiated.
A common misconception among students is that extensive properties depend on the amount of material present, whereas intensive properties do not. However, this statement is incorrect, as both extensive and intensive properties depend on the amount of material present. It's essential to understand the difference between the two types of properties to avoid confusion.
A false statement that many students might come across is that physical and chemical properties depend only on the type of matter. This statement is untrue as the properties of matter depend not just on the type of matter but also on other factors like temperature, pressure, and composition.
Another false statement that you might come across is that the density of a substance always increases as its temperature increases. This statement is incorrect as in some cases; the density of a substance may decrease with a rise in temperature. It depends on the characteristics of the substance and its conditions.
Many students might think that the hardness of a substance is an intensive property. However, this statement is incorrect, as the hardness of a substance is an extensive property and depends on the amount of material present.
Another common misconception is that all thermodynamic properties describe the equilibrium state of a system. However, this statement is false as some thermodynamic properties describe non-equilibrium states, such as the heat capacity of a system.
Finally, it's essential to understand that physical and chemical properties are not interchangeable. These two types of properties cannot be compared, and they describe different characteristics of matter.
These are some of the false statements that you might come across while studying properties in class 12 chemistry. It's crucial to identify and understand these incorrect statements to avoid confusion during exams and while studying.
In conclusion, as a student, it's pivotal to understand the properties' concept and identify the false statements one might come across while studying chemistry. Understanding these incorrect statements can help ensure that you have a better grasp of the subject and perform well in your exams. Remember, clarity of concepts can help you excel in chemistry, and it's vital to study correctly to achieve your goals.
Post a Comment for "What Are The Principles Of Electromagnetic Wave Propagation In Electrical Engineering?"